Chapter 5
The Role of Carbon Utilization
↧
Authors
CO2 utilization refers to any process that transforms captured CO2 into valuable products. Also known as carbon use, carbon recycling (or upcycling), or carbon tech, these processes strive to permanently store carbon, generate revenue, and/or avoid emissions from conventional products with large amounts of embodied carbon.
While the primary objective of CDR is net removal of atmospheric CO2 through permanent storage, there are many cases where permanence is not feasible or is too expensive. In some cases, carbon utilization may serve as an alternative pathway to permanent storage. However, it is important to note that many carbon utilization pathways do not offer permanent storage and instead may even cycle CO2 back into the atmosphere on short timescales.
Given the lack of economic incentive today to store CO2 permanently deep underground, utilization of captured CO2 as a feedstock for a product that has value, like fuel, chemicals, plastics, or concrete, could make its capture more economically feasible. Policies such as the 45Q tax credit or LCFS (see glossary for more information and references) can also help incentivize utilization. This chapter provides an overview of how carbon utilization operates today and highlights future utilization opportunities that could play a role in CDR.
5.1
5.1 —
How CO2 is sourced today
The current scale of global CO2 utilization is very limited – around 180 MtCO2/yr (Zhang et al., 2020). This represents roughly 0.5 percent of 2018 global CO2 emissions (total energy emissions were roughly 33 GtCO2 in 2018). Some projections state that 700 MtCO2/yr can be utilized by 2050 (Mac Dowell et al., 2017), while more optimistic projections envision gigatonne-scale utilization over the same time period (Hepburn et al., 2019).
The manufacture of urea (a building block of fertilizer) uses more CO2 than any other industry, accounting for nearly 65 percent of all CO2 use (Kleij et al., 2017). However, this CO2 is considered captive; in other words, it is self-supplied from the on-site capture of CO2 generated from hydrogen production, which is a primary feedstock for ammonia production. The major hydrogen production method used globally reforms natural gas with steam (known as steam-methane reforming), which produces CO2 as a byproduct at relatively high purity (Ligouri et al., 2020). This is different from merchant CO2, which is captured for the purpose of off-site distribution. In fact, in most other cases where CO2 is required as a feedstock, it is not available on site and must be purchased from the merchant market. The urea produced releases CO2 back into the atmosphere upon field application, and thus offers no long-term storage. This means that urea production can be, at best, carbon-neutral, if the CO2 is originally sourced from the atmosphere, rather than from the avoided CO2 emissions of hydrogen production from natural gas. Consider the chemical reaction of CO2 and ammonia (NH3) to make urea (CH4N2O):
5.1 →

Here, one molecule of CO2 yields one molecule of urea. Upon application, urea breaks down by the reverse reaction:
5.2 →

Thus, one molecule of urea yields one molecule of CO2. Such relationships, as determined by chemical stoichiometry, place real-world limitations on the impact of certain utilization pathways on CDR. In order for such pathways to be carbon-neutral, all material and energy flows going into the system must be carbon-neutral. Due to the complexity of ensuring all flows are carbon-neutral, making products with CO2-to-chemicals or CO2-to-fuels processes creates a smaller carbon footprint than making the same products with fossil feedstocks, with the co-benefit of decreasing our reliance on fossil-based products.
Currently, the greatest demand for merchant CO2 comes from enhanced oil recovery (EOR), mainly in the U.S. In EOR, CO2 is used as a physical solvent, which helps increase oil production by pressurizing trapped oil and lowering its viscosity, making it easier to extract. Because CO2 injection is expensive, EOR operators have tried to minimize the amount of CO2 required. To further reduce costs, nearly 80 percent of the CO2 used in EOR is derived from natural reservoirs: deep geological reservoirs called “domes” (Kuuskraa and Wallace., 2014; IEA., 2009; Kallahan et al., 2014). The CO2 is mined and transported through a network of nearly 4,000 miles of pipelines. It is widely used because it is a high-volume, low-cost source of CO2.
5.2
5.2 —
Relationship between CO2 utilization and CDR
Chapter 4 presented LCA results of a solvent-based DAC facility configured with various energy sources, with each scenario resulting in a different amount of net-removed CO2. In a similar fashion, the choice of utilization pathway – and management of that pathway – can greatly influence a CDR project’s overall effectiveness at removing CO2 from the air, in addition to the permanence of its removal. In fact, some pathways may never achieve CDR, no matter how well they are managed. This section walks through several examples that illustrate the implications of utilization on overall life cycle emissions and their ability to achieve CDR.
5.2.1
CO2 utilization in EOR
Throughout the injection and flooding of an oil field, recovery and processing of the oil and gas mixture, and subsequent re-injection of separated and recycled CO2, nearly all of the CO2 used in EOR remains sequestered deep below the Earth’s surface (Núñez-López et al., 2019). There are a number of operating parameters (e.g., project length, the technology used to separate CO2 from produced oil) that may influence how much CO2 can be stored. However, the historical average amount of CO2 permanently stored in the earth per barrel of oil (bbl) produced is roughly 0.5 tonnes. In practice, this storage, termed “associated storage,” is highly variable over the duration of the project, and the highest rate occurs in the first several years. An EOR operator may also choose to maximize oil recovery by sending excess recycled CO2 to an adjacent field, or maximize storage by re-injecting the excess CO2 into a geologic reservoir.
Globally, an estimated 40 MtCO2 (anthropogenic) are reliably stored in the earth each year, with over 90 percent of storage projects associated with EOR. Most EOR activity takes place in the United States, with other projects in Canada, Brazil, Turkey, China, Norway, Saudi Arabia, UAE, and Malaysia (Verma, 2015; Global CCS Institute, 2019; Sweatman et al., 2011; IEA, 2015; Kuuskraa and Wallace, 2014).
Figure 5.1 shows four scenarios of how CO2 could be used for EOR. Of the 68 MtCO2 used in the U.S. per year for EOR, roughly 60 Mt is sourced naturally from the earth. The remaining 8 MtCO2 are supplied anthropogenically from a mix of sources, primarily natural gas processing and to a lesser extent bioethanol production and the exhaust streams of fossil fuel combustion or gasification (State CO2-EOR Deployment Work Group, 2017; Irlam, 2017; EPA, 2018; Global CCS Institute, 2019). In the case of natural gas processing, the natural gas recovered from an underground geologic formation is often mixed with CO2 and H2S. When the natural gas is recovered at the surface, the acid gases (CO2 and H2S) must be separated before pipeline transport of natural gas. The CO2 separated in the natural gas recovery process is not conventionally included in the carbon footprint of the natural gas, but it is important to note that it would not be produced in the absence of natural gas recovery. Since natural gas requires purification for pipeline transport, the cost of the CO2 separation is embedded in the recovery costs of the natural gas. In both cases (Figures 5.1a and b), the CO2 used for EOR is simply “moved” from one location underground to a different location underground, resulting in oil production, and in some cases combined natural gas and oil production.
5.1 →
An overview of the various sources of CO2 for EOR in the US, with the pie chart representing the distribution of CO2 sources used for EOR today (Global CCS Institute, 2019): a) natural CO2 domes in the earth, b) “natural” CO2 sourced from natural gas recovery, c) avoided CO2 from fossil fuel combustion (EPA, 2018), and d) CO2 separated from the fermentation process of making bioethanol (State CO2-EOR Deployment Work Group, 2017). The bottom image, (e), depicts a pathway that could result in CDR if more CO2 is stored in the earth than produced as a result of the LCA of the recovered oil.
An overview of the various sources of CO2 for EOR in the US, with the pie chart representing the distribution of CO2 sources used for EOR today (Global CCS Institute, 2019): a) natural CO2 domes in the earth, b) “natural” CO2 sourced from natural gas recovery, c) avoided CO2 from fossil fuel combustion (EPA, 2018), and d) CO2 separated from the fermentation process of making bioethanol (State CO2-EOR Deployment Work Group, 2017). The bottom image, (e), depicts a pathway that could result in CDR if more CO2 is stored in the earth than produced as a result of the LCA of the recovered oil.
As shown in Figure 5.1c, when CO2 is captured at a point source (such as a fossil-fueled power plant) and used for EOR, the CO2 emissions associated with the combusted fossil fuel (coal or natural gas) are avoided since they are stored back in the earth via EOR. In this case, the cost of separating CO2 from the power plant may be incorporated into the cost of the electricity generated by the power plant, which would result in the plant producing electricity with a lower carbon intensity. The use of this avoided CO2 for EOR, however, does not impact the carbon intensity of the oil recovered from the enhanced recovery process. Any “credit” associated with avoiding CO2 emissions into the atmosphere can be counted only once, i.e., to offset the carbon intensity of the fossil-based electricity or the crude oil being recovered.
Figure 5.1d shows the scenario where CO2 is sourced from the fermentation process of converting biomass to ethanol, which produces CO2 at a purity of 98 percent or even more. To accurately determine the reduced carbon footprint of bioethanol compared to fossil-based ethanol, one must account for all of the CO2 emissions associated with the energy required for the fermentation process, in addition to the embodied carbon in the materials and infrastructure of the biomass conversion process. Some of the carbon from the biomass is associated with the bioethanol fuel, and some is associated with the separated CO2 that is available for utilization. The LCA tools outlined in Chapter 4 provide a framework for determining the reduction potential of this route compared to conventional processes. For this route to qualify as CDR, there must be more CO2 stored underground than is generated through the process of biomass conversion through its utilization.
Figure 5.1e shows a scenario where CO2 for EOR comes purely from the atmosphere. Strictly in terms of carbon accounting, this combined scenario can result in CDR if more CO2 is stored in the earth than is produced in the oil recovery process. When considering that fuel combustion releases CO2 at a ratio of approximately 73 grams (g) CO2/MJ fuel lower heating value (LHV) and approximating a barrel of oil at 5.8 GJ fuel LHV (diesel), at least 0.42 tCO2/bbl must be utilized and stored to account for combustion emissions alone. Adding the remaining emissions associated with processing, transport, crude oil refining, and any other upstream or downstream life cycle emissions, even best-practice EOR operations are more carbon-intensive than the aforementioned conventional production of diesel fuel (0.50 – 0.51 tCO2eq/bbl), and under typical conditions, emissions are roughly 0.59 tCO2/bbl. Thus, an operator would need to utilize 0.59 tCO2/bbl from the atmosphere for the EOR to be considered carbon-neutral. Since the average amount of CO2 stored over the life of an EOR project is 0.5 tCO2/bbl, offsetting the emissions associated with the recovery, refining, transport, and oxidation of the fuel would require roughly an additional 0.1 tCO2/bbl for dedicated storage. Also, critically, while this combined scenario can leave more CO2 underground than is emitted in fuel production and consumption, it inevitably leads to the production of “new” carbon dioxide from the burning of fossil fuels, which may not have otherwise entered the atmosphere. The potential systemic impacts of this activity on fossil fuel consumption must be considered alongside the carbon accounting.
In practice, several additional parameters dictate the actual carbon footprint for EOR, including oil field characteristics (e.g., the injection pressure needed to recover oil, because higher injection pressures require more energy, and thus burn more fossil fuels), the carbon intensity of the electrical supply (grid) required to support CO2 compression, separation of the recovered oil and CO2 mixture, upstream and downstream transport, and whether other products are recovered. For instance, gasoline is more carbon-intensive to produce than diesel, and many EOR operations also yield feedstocks for non-fuel-based products like chemicals. While these decisions can have a moderate impact on lifecycle emissions, on the order of 6 – 10 percent (Cooney et al., 2015), the overall carbon intensity is ultimately most sensitive to the CO2 utilization rate. A higher CO2 utilization rate means more CO2 stored per unit of oil recovered.
Coupling DAC to utilization of any form can assist in financing its deployment today. However, when coupling DAC to EOR, volatility in the price of oil can lead to vulnerabilities in financing DAC projects. Although not a DAC project, the point-source capture project Petra Nova recently shut down due to the precipitous drop in oil prices that made the cost of CO2 separation from the power plant too expensive (International CCS Knowledge Centre, 2020). Sourcing CO2 from DAC and other point-source capture projects can become non-viable when the project costs ($70 – $100/tCO2 for point-source capture and $250 – $600/tCO2 for DAC) exceed those of a facility with CO2 sourced from underground (Rubin et al., 2015; Psarras et al., 2020; NASEM, 2019; McQueen et al., 2020).
5.2.2
Use of CO2 as a feedstock for chemicals and fuels
Several chemical routes are the basis of profitable industries, including production of fuels, organic carbonates, methanol, urea, and aliphatic/olefinic hydrocarbon chains. Due to the strength of the carbon-oxygen double bond, the thermodynamics of chemical CO2 conversion are unfavorable. Hence, several different methods can be used to facilitate efficient CO2 conversion, including combination with high-energy reactants such as H2 or strained-ring epoxides, shifting the chemical equilibrium by removing water from the products or co-producing water in the reaction, or adding energy to the reaction in the form of electricity or light. The energy source and amount of energy associated with the conversion of CO2 or preparation of high-energy reactants such as carbon monoxide and hydrogen have a significant impact on the carbon footprint of the process. It is important to understand how the CO2-derived product pathway compares to conventional production pathways. Take, for example, the production of methanol from carbon oxides:
5.3 →

5.4 →
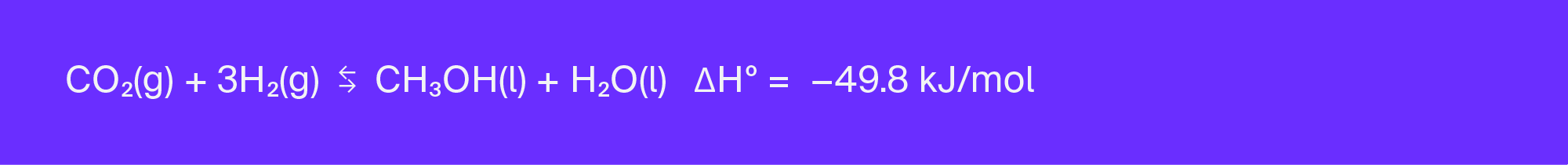
The feedstocks (CO and hydrogen gas, or H2) of Equation 5.3 are collectively termed “synthesis gas,” and are produced during gasification (reacting fossil fuels or biomass at high temperatures without combustion). Methanol (CH3OH) has been produced this way for decades (U.S. EIA, 2019; Hydrocarbon Processing, 2020a, 2020b), and there are many plants coming online globally that can produce thousands of tonnes of methanol per day.
By contrast, the reaction pathway shown in Equation 5.4 uses CO2 as a feedstock. The primary difference between these two methanol production pathways is the relative amount of CO2 and H2 required for the reaction, based on their differing stoichiometry. An additional 0.06 tonnes of hydrogen gas is required for the direct conversion of CO2 to 1 tonne of CH3OH (Equation 5.4) when compared to the conventional route (Equation 5.3). Today, 95 percent of the H2 produced globally comes from steam methane reformation (SMR) (Rapier, 2020), where roughly 9 tonnes of CO2 are generated for every tonne of H2 produced (Sun and Elgowainy, 2019). Thus, the direct conversion of CO2 to CH3OH yields an additional 0.54 tonnes of CO2 in emissions for every tonne of CO2 utilized. Lowering the carbon intensity of hydrogen production is thus vital to reducing methanol’s carbon footprint.
“Blue” hydrogen generation refers to steam methane reforming (reacting methane under pressure with steam to produce hydrogen) with carbon capture to reduce direct CO2 emissions. Steam methane reforming with carbon capture can theoretically reduce emissions by up to 100 percent, but is closer to 70 percent in practice (Ligouri et al., 2020). “Green” hydrogen is produced through the electrolysis of water, preferably powered by renewable, low-carbon energy. The choice of energy source is even more important here, as electrolysis is highly energy-intensive (between 50 and 60 MWh/tH2) (National Renewable Energy Laboratory [NREL], et al., 2018). Understanding how CO2 utilization impacts production is particularly important for inputs with sizable carbon footprints. It is equally vital to carefully consider how low- or zero-carbon energy sources are used, and to prioritize them for purposes that maximize carbon reduction, at least while such energy sources are in short supply. With respect to the stoichiometry of carbon dioxide, 1.38 tonnes of CO2 is “fixed” in each tonne of CH3OH produced. However, any CO2 fixed within methanol is released back into the atmosphere on a short timescale (days to weeks after its combustion as a fuel, or up to decades if used as a precursor to form chemicals like formaldehyde, acetic acid, and methyl tert-butyl ether). Using a standard emission factor associated with methanol as a fuel , operational use of one tonne of methanol results in 1.55 tonnes CO2eq released into the atmosphere (Argonne National Laboratory, 2020). If this carbon came from a fossil fuel (i.e., the CO2 utilized was captured from a fossil fuel-fired power plant or industrial facility), these emissions result in a 1.55-tonne increase in atmospheric CO2 per tonne CH3OH produced. If, however, the CO2 came from the atmosphere, the net emissions are lowered to 0.17 tonnes CO2 per tonne CH3OH produced.
The total effects of these considerations are outlined in Table 5.1 using “gray” hydrogen derived from SMR without carbon capture as an example of a poorly managed process, and green hydrogen derived from solar-powered electrolysis as an example of an optimal process. Note that the carbon intensity of solar electricity will continue to decline with increased use of carbon-free energy in its manufacturing process.
5.1 →
The best-case scenario presented is direct hydrogenation of ambient CO2 (from the atmosphere) coupled with green hydrogen, yielding 0.42 tonnes CO2eq per tonne of methanol produced. However, this scenario still results in net positive emissions to the atmosphere. At best, the amount of CO2 fixated in a product can exactly offset the amount released upon consumption or breakdown, leading to net-neutral emissions. In reality, there are several additional steps (e.g., processing, transport) that lead to incremental emissions and net emissions to the atmosphere. However, replacing fossil fuel-sourced carbon with atmospheric carbon can still result in significant emission reductions. While these reductions are not negative emissions, they can play an important role in mitigating climate change. An additional route, still nascent in this field, is sourcing hydrogen from biomass waste gasification coupled with reliable storage of the generated CO2. This pathway has the potential to produce “negative hydrogen,” which when coupled with CO2 sourced from air has the potential to produce fuel with a negative carbon footprint and thus result in CDR.
Methanol is a flexible intermediate in that it can be used to produce ethylene and propylene, which in turn are feedstocks for plastics, coolants, and resins. Methanol can also be used as an intermediate in the production of gasoline, diesel, soaps, and cleaning fluids (Olah, 2005). Furthermore, since methanol is a liquid at room temperature, its transport is less energy-intensive than compressing and transporting CO2, and it is safer to handle than pressurized hydrogen. Several approaches exist today at varying stages of development (Opus 12, 2020; Prometheus, 2020; De Luna et al., 2019) that use low-carbon energy (or in some cases photons, or light energy) to catalytically react CO2 and hydrogen together to create synthetic fuels and chemicals (Lewis, 2016; Mckone et al., 2014; Walter et al., 2010; Wilcox, 2012). Advancing the production of synthetic fuels, chemicals, and products using CO2 as a feedstock helps create an alternate pathway for meeting the demand for these consumer products, without the need for crude oil.
5.2.3
CO2 utilization for concrete coupled with low-carbon cement
The cement sector is responsible for roughly 7 percent of global CO2 emissions, and cement production is anticipated to grow 12 – 23 percent by 2050 (Fernandez pales and Leung, 2018). Cement is a major component of concrete, the most widely used man-made material in the world; thus, reducing emissions in the cement sector might be achieved through reduced concrete use and/or incorporation of captured CO2 directly into the concrete mixture (Huang et al., 2019, Woodall et al., 2019). Typical concrete has a volumetric composition of 60 – 75 percent aggregate, 7 – 15 percent cement, 14 – 18 percent water, and up to 8 percent air (Huang et al., 2019). Synthetic aggregate can be formed from the direct reaction between carbon dioxide and an alkaline source to yield solid carbonates (Kurda et al., 2018). This process has three advantages over conventional concrete production. First, synthetic aggregate can replace a portion of other coarse and fine aggregates (e.g., sand and dolomitic limestone), potentially reducing emissions associated with material transport and handling. Second, synthetic aggregate is often less dense than non-synthetic aggregates, which can make the resulting concrete blocks less dense, as well. This can reduce costs by lowering the amount of cement required to make the equivalent number of concrete blocks for a building project (assuming blocks built with synthetic aggregate have the same mechanical strength). Finally, carbonates formed from atmospheric carbon have the potential to achieve carbon dioxide removal, contingent on a full LCA (Huang et al., 2019). A rough breakdown of the carbon footprint of conventional concrete is 113, 13, 0.6, 0.01, and 4.65 kgCO2/t concrete for cement, gravel, sand, water, and mixing, respectively, totaling a conventional concrete carbon intensity of roughly 131 kgCO2/t concrete (Kurda et al., 2018).
Several companies in the building materials sector are actively pursuing low-carbon cement and/or concrete production (Blue Planet, 2019; CarbiCrete, 2020; CarbonCure, 2020; Solidia, 2020). Critically, their sources of CO2 today are often from industrial waste streams, and thus count as avoided emissions; any “credit” associated with these efforts cannot be counted twice. For instance, if a cement producer obtains CO2 from the exhaust of a natural gas-fired power plant, the plant could claim credit for selling low-carbon electricity, or the cement producer could claim credit for utilizing and storing the CO2 – but not both! The CO2 avoided can be counted only once.
To illustrate this concept, we will describe several potential approaches for producing carbon-negative concrete. Each approach is effective, but would be especially powerful in combination with the others, illustrating how a combination of approaches may be required for utilization to result in CDR. The three approaches are:
Capturing and storing carbon from the exhaust of the cement kiln;
Replacing gravel and sand with synthetic aggregate that stores CO2 from DAC; and
Replacing fossil fuels used in the kiln for clinker production with biomass, coupled with carbon capture and storage.
Here we describe each approach in more detail. Figure 5.3 demonstrates the carbon intensity of concrete and how each approach can reduce concrete’s carbon footprint and improve the potential of carbon production. Figure 5.3a shows the conventional carbon intensity of 131 kgCO2/t concrete, compared to 5.3b, where, when coupled with point-source capture at the kiln of the cement plant, the carbon intensity is reduced to 29 kgCO2/t concrete. Finally, Figure 5.3c shows several removal approaches being applied, to increase the net removal of CO2 from the atmosphere to 116 kgCO2/t concrete. This analysis serves as a best-case scenario since it assumes that all energy used within each process is carbon-free, from the process itself to the fuels used in the transportation of feedstocks or products.
1. Carbon capture and storage from the cement kiln
Conventional cement (e.g., ordinary Portland Cement) has a carbon intensity of about 850 kgCO2/t cement, most of which results from calcining limestone to produce clinker (approximately 750 kgCO2/t of cement). The remaining 100 kgCO2/t of cement has a mixture of sources, including indirect emissions associated with the extraction of raw materials (such as the limestone) and the fuels burned in the kiln. Retrofitting the kiln with carbon capture technology at 90 percent reduction results in a carbon intensity of 675 kgCO2/t cement. This is a roughly 79 percent reduction of the carbon footprint of conventional cement. Since cement comprises about 15 percent of the concrete, this technology could reduce CO2 emissions by roughly 101 kg/t of concrete.
2. Synthetic aggregate coupled to DAC
Technologies that can make synthetic sand and gravel for concrete production, using an alkalinity source and mineralizing with CO2, already exist (Ando, 2020; Huang et al., 2019; Blue Planet, 2019). Related technologies can also produce concrete using less clinker and cure it with CO2 (CarbonCure Technologies, 2020; Solidia, 2020). These technologies vary in their ability to store CO2 in concrete. It was recently reported that up to 100 kgCO2 could be stored per tonne of concrete (Blue Planet, 2019). Sourcing CO2 from the air (DAC) is costly today, but carbon-negative concrete approaches could provide the scale (MtCO2 removal per year) for increased deployment in DAC, facilitating “learning by doing” and driving down costs. On average, a cement plant with a carbon footprint of roughly 1 MtCO2/yr produces roughly 1.2 Mt cement per year. As mentioned above, cement represents 15 percent of the concrete by weight, so this equates to making roughly 13 Mt of concrete per year, assuming all of the cement goes to making concrete. Using the estimate of 100 kgCO2 stored per tonne of concrete, this indicates that 1.3 MtCO2/yr from DAC is required to produce synthetic aggregate to replace sand and gravel in the concrete formulation. This scale of CO2 demand would couple especially well with DAC technologies that benefit from economies of scale, such as Carbon Engineering’s solvent-based approach (Keith et al., 2018).
3. Displacement of fossil fuels with waste biomass-based fuel
Not all biomass waste is created equal. For instance, shells from almonds and pistachios or fruit pits can be fired with other fuels in cement kilns and are usually sourced as a local waste. These particular biomass wastes are very carbon-dense and can serve as a drop-in fuel for coal, which currently represents 60 percent of the fuel burned in cement kilns in the U.S. Since the cement kiln exhaust would be retrofitted with carbon capture, this would count as avoided emissions. However, burning the biomass waste would be considered carbon dioxide removal since it is not a fossil fuel and the organic material itself has taken CO2 from the air. The net removal associated with use of biomass depends on the CO2 emitted from transport and any required pre-processing of the biomass to serve as a drop-in fuel, whether it is natural gas, coal, or diesel.
Co-firing with nut shells is current practice in cement facilities in Arizona (pecan shells), Southern California (pistachio shells), Florida (peanut shells), and Texas (pecan shells) (Demirbas, 2006; Erol et al., 2010). The heating value of the raw unprocessed shells ranges from 17 to 21 MJ/kg, which is in line with lignite and some sub-bituminous coal. Minimal pyrolysis achieves increased heating values that range from 28 to 31 MJ/kg, which is in line with higher-ranked coals such as bituminous and anthracite (Edgar, 1983). The fuels burned in U.S. cement kilns today are a mix, but 60 percent rely on coal with roughly 50 percent lignite and sub-bituminous and the other 50 percent bituminous, indicating that this high-quality biomass could be used as a drop-in fuel for coal.
Assuming a 50/50 split in weight between coal and diesel co-fired in the cement kiln, and further assuming a carbon footprint for clinker production of 750 kgCO2/t clinker (with calcining comprising ~ 55 percent of the CO2 exhaust and the fuel), the remaining 45 percent leads to a carbon footprint of ~ 202.5 kgCO2/t clinker for the coal. This also assumes that the carbon intensity of the diesel is roughly 60 percent that of coal. (Natural gas would be roughly half.)
If the coal were displaced with high-quality waste biomass, such as nut shells with an equivalent heat value and carbon density, this could result in ~ 27 kgCO2 avoided and removed per tonne of concrete since using the biomass waste as a drop-in fuel for coal coupled to carbon capture is CDR.
Currently, several companies use biomass waste to produce diesel oil. These include NuFuels, Clean Energy Systems, and Charm. Again, assuming that 50 percent of the fuel burned in the kiln is diesel, this would result in 135 kgCO2/t clinker. Replacement of this conventional diesel with biodiesel would result in 18.2 kgCO2 avoided and removed per tonne of concrete since the biodiesel is CDR.
Combining all three approaches could yield a total carbon dioxide removal potential of 116 kgCO2/t of concrete produced. This is almost equal and opposite the conventional approach of producing concrete today, which has an estimated footprint of 131 kgCO2/t of concrete (Figure 5.2).
5.2 →
Combining CCS, synthetic aggregate using DAC, and fuel replacement with hiqh-quality biomass waste and biodiesel results in a maximum CO2 removal of 116 kgCO2/t concrete. This is nearly equal and opposite to the conventional approach (above left), which emits approximately 131 kgCO2/t concrete.
Combining CCS, synthetic aggregate using DAC, and fuel replacement with hiqh-quality biomass waste and biodiesel results in a maximum CO2 removal of 116 kgCO2/t concrete. This is nearly equal and opposite to the conventional approach (above left), which emits approximately 131 kgCO2/t concrete.
This example remains hypothetical – any actual implementation would require many more details and would likely include several additional caveats beyond the carbon accounting. However, the example illustrates the potential scale of CDR that can be achieved through a practical, real-world utilization pathway.
5.3
5.3 —
The scale of carbon utilization
The scale of CDR required to make an impact on climate change – 10 GtCO2/yr by midcentury and 20 GtCO2/yr by 2100 (Chapter 1) – would result in a supply of CO2 that could quickly overwhelm CO2 demand, which is roughly 80 Mt/yr in the U.S. and 180 Mt/yr globally (Zhang et al., 2020). Current CO2 markets would need to grow by a factor of 10 to 100 to match that supply. There is competition for carbon utilization opportunities in the form of incumbent CO2 providers, for which CO2 purchasing contracts already exist, as well as from other mitigation strategies, namely point-source CCS. Thus, alternative uses and markets for captured CO2 will likely be necessary. This includes making materials from CO2 that are currently made with fossil carbon and finding new uses for CO2 in building materials.
Several routes for the transformation of CO2 into a useful product are described in Table 5.2, along with a projected utilization potential by midcentury (Callahan et al., 2014; IEA, 2019; Kuuskraa et al., 2011; Hepburn et al., 2019; IFA, 2017; IEA, 2007; Campbell, 2018; Milani et al., 2015; EIA, 2019; EPA, 2018). In many processes, CO2 is used directly without the need for conversion. For example, in the first commercial DAC facility in Hinwil, Switzerland, Climeworks captures CO2 directly from the air and sends it via pipeline to an adjacent greenhouse, where the CO2 acts as a nutrient, boosting crop yields by more than 20 percent before the CO2 is vented back to the atmosphere (Climeworks, 2020). In other cases, such as synthetic fuels, chemicals, plastics, and carbonates, CO2 is transformed via chemical reactions to form products in which the carbon is embedded.
5.2 →
Market competition with incumbent processes continues to stall scaling utilization. This challenge motivates understanding where CDR paired with utilization can be profitable and thus attract more investment (Markit, 2016). Industry analysis shows that the market value of delivered CO2 ranges from $44 to $660 per Tonne, with high-purity CO2 delivery at ISBT specifications (99.9 percent CO2) suitable for food and beverage use commanding higher prices. Incentives like the U.S. California LCFS or 45Q tax credit can defray CDR costs.
Local markets for utilization products could create regional demand for CDR projects. Unlike CCS, CDR projects are not necessarily tethered to point source emissions and/or suitable sinks, making them more flexible in regions where other CO2 supplies are not practically available. Indeed, the existence of nearby utilization markets could serve as a siting consideration when planning CDR deployment strategies. The IHS Markit report (2016) on industrial costs of bulk delivered CO2 notes that buyers in some regions pay more for CO2 due to high transportation costs. These regions could represent opportunities for local CDR operations to act as CO2 providers and gain access to utilization markets.
Not all utilization pathways are mutually exclusive, and they face market competition. For example, advancement of low-carbon synthetic fuel production could decrease the demand for EOR, and vice versa. Likewise, increased use of cross-laminated timber (prefabricated wood panels) could displace a portion of the market for concrete and steel building materials (Song et al., 2018). Ultimately, the extent of utilization (and the choice of pathway) depends on the agent of financial and other regulatory support (e.g., building codes, government use of fuels, and materials).
5.4
5.4 —
Timescales of storage in carbon utilization
As described in Chapter 4, CDR requires CO2 to be stored or used in an effectively permanent form. Deep and dedicated geologic storage and proper post-injection monitoring clearly achieves such permanence. The effective permanence of carbon utilization pathways is often less clear, but there are examples of effective permanent storage, such as utilization for manufacture of aggregates and concrete materials.
In general, we can consider time relative to the moment a CO2 molecule is removed from the atmosphere, which we refer to as t = 0. For a technological system like DAC, t = 0 is the moment ambient CO2 reacts with the basic solvent or sorbent; for a biological system it might be photosynthetic uptake. In either case, at t = 0, the CO2 molecule is in a non-atmospheric subsystem. The purpose of geologic storage is to extend t into meaningful timescales, i.e., t > 100 years, according to the IPCC (2014), and perhaps well beyond.
What happens with carbon utilization pathways? If CO2 is used to make short-lived products like industrial chemicals and solvents, then CO2 is released upon degradation or decomposition. This may occur in a few weeks or even sooner, and t rarely lasts longer than a few years. For the case of urea described in Section 5.1, for example, the compound becomes hydrolyzed upon broadcast, releasing CO2 on the order of t = six months.
On the opposite end of the spectrum, CO2 may be stored in a subsystem for very long periods, either by being absorbed into subsurface pores or through chemical transformation into a stable form from which it cannot be re-released (e.g., carbonate or plastic).
5.5
5.5 —
Assessing risk
That CDR will enable business as usual to continue is a common concern. Carbon utilization specifically may dissuade key actors from pursuing systemic changes to mitigate climate change. As illustrated throughout this chapter, many utilization pathways result in the short-term release of CO2. Use of less effective pathways early on could lead to market-driven technological lock-in, making it difficult and expensive to escape. Locking into pathways that provide minimal or no CDR could be detrimental to broader CDR efforts.
Another risk associated with carbon utilization is leakage, or the shifting of emissions elsewhere as a result of climate policy. As an example, leakage occurs in forest carbon offset credit programs when a reduction in timber harvesting at a project site, in exchange for emissions reductions or carbon dioxide removal credits, causes timber harvesting to increase somewhere else to meet demand. Another example is leakage in the EU power sector: Emission control drives up the cost of power locally. This, in effect, makes other power generators (those that are not subject to the same constraints) competitive. Likewise, a business may choose to relocate to a region with less stringent climate policies. Either case could result in increased emissions outside of the constrained region. While the EU recognizes both the cement and ammonia sectors as high-risk sectors for leakage, Naegele and Zaklan (2019) found no evidence of carbon leakage in EU manufacturing. Nonetheless, leakage can occur whenever climate policy varies regionally and impacts commodity costs unequally. To maximize CDR potential, it is important to identify at-risk sectors and pathways and provide proper political support, analysis, and oversight to mitigate leakage.
Today, nearly every technological CDR system that captures CO2 from the air has a carbon utilization partner with projects that do not include permanent storage. As mentioned earlier, Climeworks’ first plant in Hinwil removes 900 tonnes of CO2 per year and feeds it via pipeline to an adjacent greenhouse facility. Of their 15 DAC facilities planned or installed, 13 are paired with utilization, including use of carbon dioxide in greenhouses as a fertilizer, for beverage carbonation, or fuel production. Global Thermostat, which employs a DAC technology based on solid sorbent capture, seeks similar carbon customers to purchase captured CO2, and Carbon Engineering (2019) has explored the conversion of its captured CO2 into transportation fuels.
5.6
5.6 —
Conclusions
Carbon utilization may provide economic incentives for CCS or CDR projects while simultaneously storing CO2 on various timescales. Compared to CO2 storage without utilization, several potential benefits of utilization should be clear: the ability to produce valuable products, additional sources of funding or incentives to scale-up removal technologies, and the displacement of fossil fuel-sourced products with ones that use CO2 and carbon-neutral hydrogen as feedstocks (e.g., chemicals, fuels, and plastic). But utilization also creates potential challenges and concerns, including CDR that is less effective when considering the complete life cycle of the utilization pathway, CO2 storage that is not permanent, and the potential to continue enabling systems that contribute to fossil-fuel emissions.
It might appear that increased demand for CO2 utilization could create competition between sources, in particular, between atmospheric CO2 via DAC and point-source capture. However, as we hope this chapter has illustrated, creative industrial design can bring together point-source carbon capture with DAC in the same utilization approach, and avoiding carbon and carbon dioxide removal can work in tandem rather than competitively to achieve maximum impact.
68.09 gCO2eq per MJ CH3OH, and 22.7 MJ per kgCH3OH
1.55 tonnes CO2 (emitted in combustion) – 1.38 tonnes CO2 (removed from the atmosphere) = 0.17 tonnes net
Assumes 55 MWh/kg H2 produced and 25 kg CO2/MWh carbon intensity of solar electricity
In approach (3), the form of biomass used to displace the fuel will depend on the fuel it replaces in the kiln. For instance, three different biomass fuels – H2 sourced from biomass, carbon-dense nut shells, and biodiesel – may be used to replace natural gas, coal, and diesel, respectively.
The LCFS awards project-based credits per tonne of CO2 removed for DAC/EOR operations anywhere in the world. Synthetic fuel products made from atmospheric CO2 must be sold in California and are awarded credits against a baseline product carbon intensity.
68.09 gCO2eq per MJ CH3OH, and 22.7 MJ per kgCH3OH
1.55 tonnes CO2 (emitted in combustion) – 1.38 tonnes CO2 (removed from the atmosphere) = 0.17 tonnes net
Assumes 55 MWh/kg H2 produced and 25 kg CO2/MWh carbon intensity of solar electricity
In approach (3), the form of biomass used to displace the fuel will depend on the fuel it replaces in the kiln. For instance, three different biomass fuels – H2 sourced from biomass, carbon-dense nut shells, and biodiesel – may be used to replace natural gas, coal, and diesel, respectively.
The LCFS awards project-based credits per tonne of CO2 removed for DAC/EOR operations anywhere in the world. Synthetic fuel products made from atmospheric CO2 must be sold in California and are awarded credits against a baseline product carbon intensity.